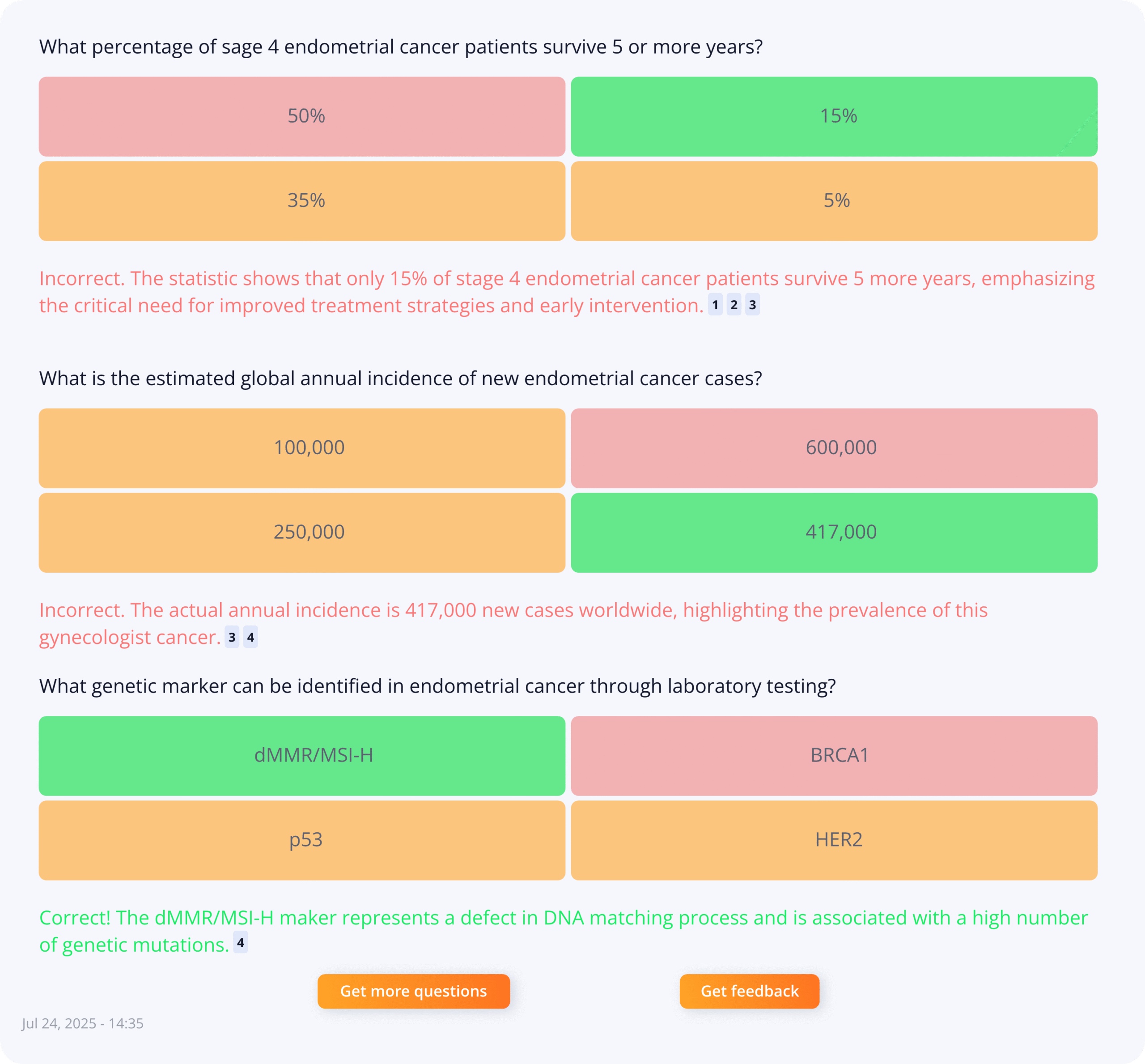
Life-science teams are under pressure to deliver systematic literature reviews (SLRs) and dossier content faster, with higher consistency and full traceability. Publication volumes keep...
Use Cases
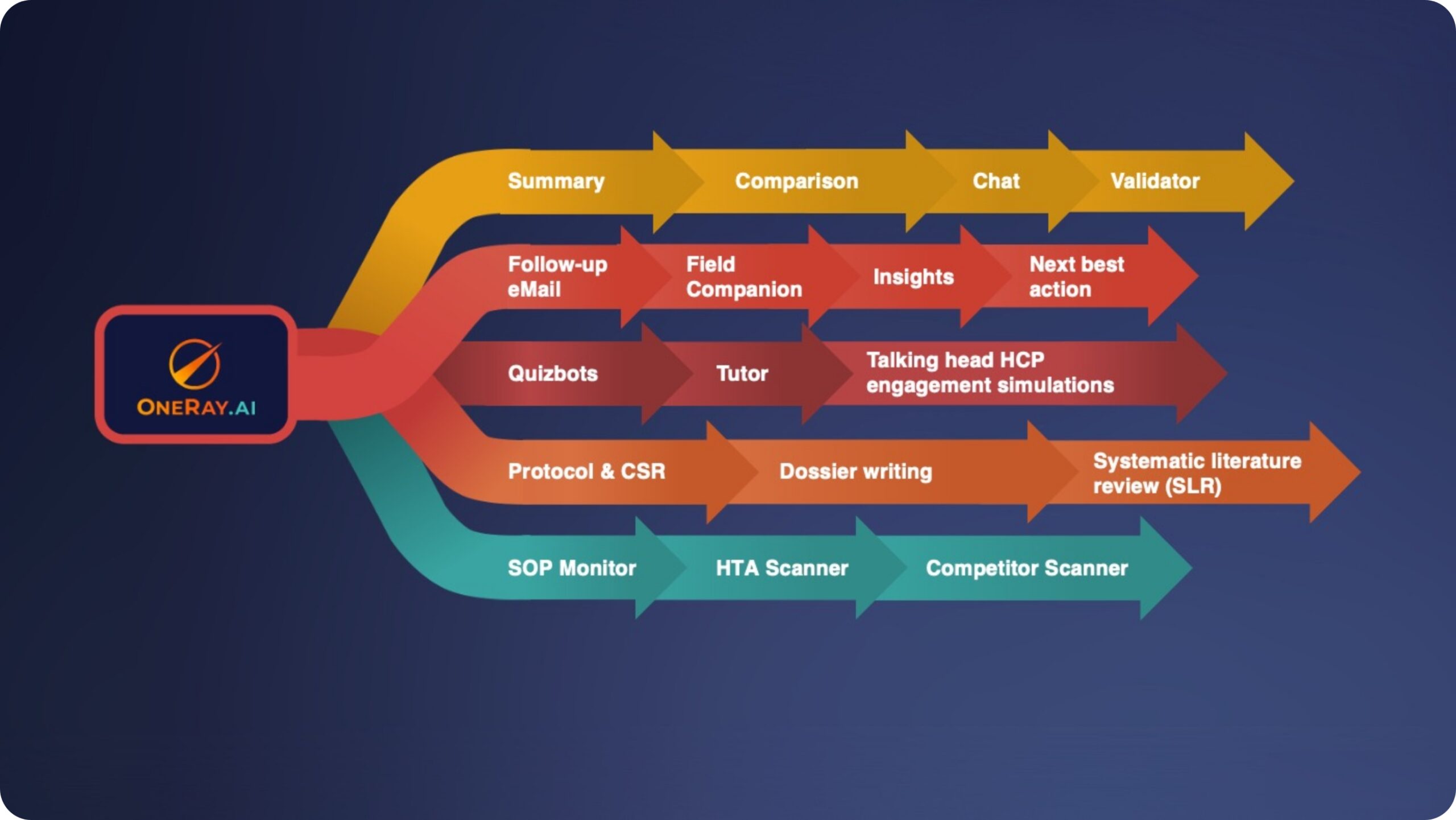
OneRay delivers robust, industry-specific solutions built around the most relevant use cases in life sciences. Our expanding ecosystem of intelligent agents is specifically designed to enhance knowledge work across pharmaceutical research, clinical development, regulatory affairs, and scientific operations. These advanced generative AI agents continuously evolve and adapt, showcasing multi-faceted capabilities that address your complex business challenges—from accelerating medical and regulatory processes to optimizing content generation and training. By focusing on real-world applications that matter most to life sciences professionals, OneRay empowers organizations to transform their knowledge work and drive meaningful innovation in healthcare advancement.
AI generated Regulatory Insights
Challenge: Time-consuming manual processing and error-prone labeling of vast regulatory data.
Solution: FFI-Ventures’ gen AI solution OneRay.ai integrates with Veeva’s RIM to automatically process and analyze regulatory documents. The system can:
- Generate concise summaries of new legislation, regulatory guidance, and Health Authority Public Assessment Reports.
- Provide specific answers to complex regulatory questions by analyzing the entire regulatory database.
- Automatically label and categorize new regulatory data, reducing human error and improving searchability.
- Create customized reports on regulatory trends and potential impacts on current products or pipelines.
This AI-driven approach significantly reduces the time and resources needed for regulatory intelligence, while improving accuracy and comprehensiveness.
AI Protocol & Clinical Study Report (CSR) Writing
Challenge: Tedious protocol and CSR writing, requiring extensive manual effort for alignment and data incorporation.
Solution: FFI-Ventures gen AI solution OneRay.ai, integrated with Veeva’s clinical data management platform, revolutionizes the protocol and CSR writing process:
- For protocols, users input essential study design elements, and the AI generates a complete protocol using the organization’s template and appropriate language from other studies and pre-clinical sources.
- For CSRs, the system automatically pulls in relevant tables, figures, and listings from the clinical database, dramatically reducing manual work and the need for quality control.
- The AI can suggest study-specific modifications based on the particular therapeutic area, patient population, and regulatory requirements.
- It ensures consistency across documents and compliance with the latest regulatory guidelines.
This approach not only saves time but also improves the quality and consistency of these critical documents.
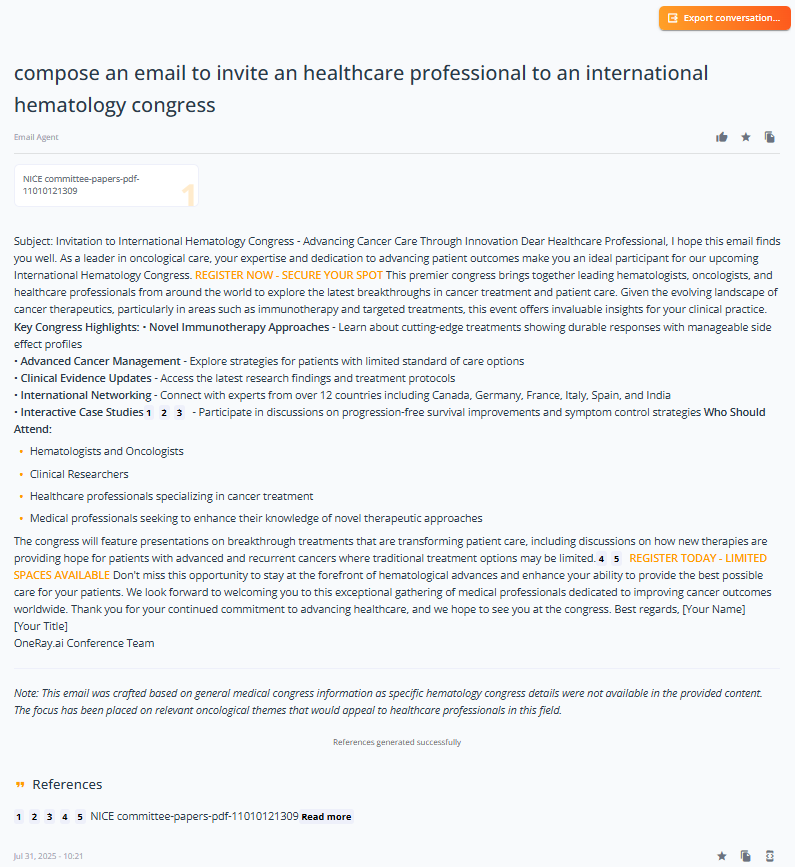
Content generation for healthcare professionals and patients
Challenge: Creating high-quality, channel-optimized content across multiple touchpoints while maintaining consistent messaging, appropriate tonality, and regulatory compliance—all within tight timelines that often bottleneck marketing and medical affairs teams.
Solution: By integrating OneRay.AI’s advanced content generation capabilities with your existing content management systems (e.g. Veeva Vault solutions), we deliver a comprehensive content optimization platform:
- The AI generates tailored content that adapts tonality, complexity, and format based on the specific communication channel—whether for tailored email communicaiton, website contents, patient education materials or in-person interactions.
- Content is dynamically optimized for target audiences, from healthcare professionals and patients to regulatory bodies, ensuring maximum engagement and comprehension.
- All generated content comes pre-equipped with accurate, traceable references and citations, significantly accelerating the Legal and Medical review process by providing immediate source verification.
- The system maintains brand consistency and regulatory compliance across all content types while reducing manual review cycles through built-in compliance checks and standardized formatting.
This intelligent content generation approach transforms content creation from a time-consuming task into a streamlined, scalable process that enhances communication effectiveness while ensuring regulatory integrity and accelerating time-to-market for critical materials.
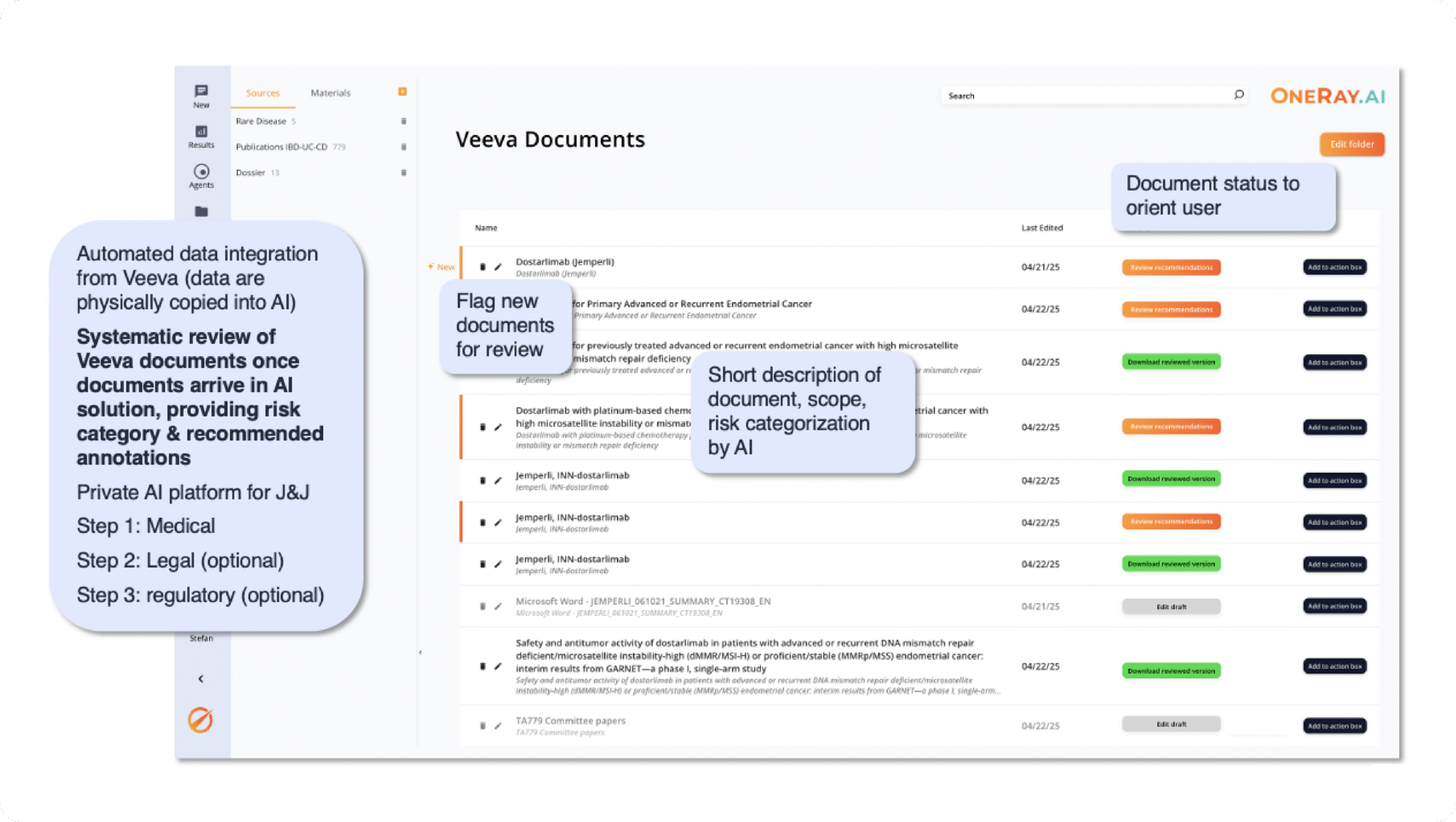
AI assisted MLR review integration into Veeva
Challenge: Medical, Legal, and Regulatory (MLR) review processes are often resource intense, with manual reviews consuming weeks of expert time while increasing risk of inconsistent feedback and delayed market access for critical materials.
Solution: By seamlessly integrating OneRay.AI’s intelligent review capabilities directly into your Veeva environment, OneRay.AI offers an automated LMR review system that accellerates approval workflows:
- The AI conducts comprehensive topic-specific annotations including Legal, Regulatory, and Medical-based reviews following your organization’s specific guidelines and instructions, with additional annotation capabilities for spelling, grammar, and style consistency.
- Advanced text-highlighting functionality enables precise identification of review comments relative to specific text passages, streamlining reviewer focus and response accuracy.
- Complete Veeva integration ensures seamless workflow continuity, automatically capturing all AI-generated annotations and user submissions while maintaining direct links to related PDF text sections.
- The system maintains complete audit trails of all annotation details and review decisions, ensuring regulatory compliance and facilitating efficient reviewer collaboration.
This integrated approach reduces LMR review cycles from weeks to days while improving review consistency and quality, enabling faster content approval and accelerated time-to-market for pharmaceutical communications and regulatory submissions.
AI guided Personalized Training & Medical Education
Challenge: Creating tailored training and educational materials for internal medical or commercial functions as well as healthcare professionals (HCPs) based on their specific needs, interests, and knowledge gaps.
Solution: By leveraging Veeva’s extensive Veeva Vault / Promomats data and FFI-Ventures’ generative AI solution OneRay, we create a personalized training and education platform:
- The AI analyzes an HCP’s interaction history, prescribing patterns, and expressed interests to generate customized educational content.
- It can create interactive training modules that adapt in real-time based on the HCP’s responses and engagement level.
- The system ensures all generated content aligns with the latest approved medical information and regulatory guidelines.
- It can suggest optimal timing and channels for delivering educational content to each HCP, maximizing engagement and knowledge retention.
This personalized approach enhances the effectiveness of medical education initiatives, ultimately improving patient care through better-informed healthcare providers.
See OneRay.AI in Action
Discover how our specialized AI solutions can transform your life sciences operations. Schedule a personalized demonstration where we’ll showcase relevant use cases specific to your organization’s challenges and objectives.
Our experts will walk you through practical applications tailored to your workflow, demonstrating how OneRay accelerates your research, streamline processes, and enhance decision-making across your organization.
FURTHER TOPICS
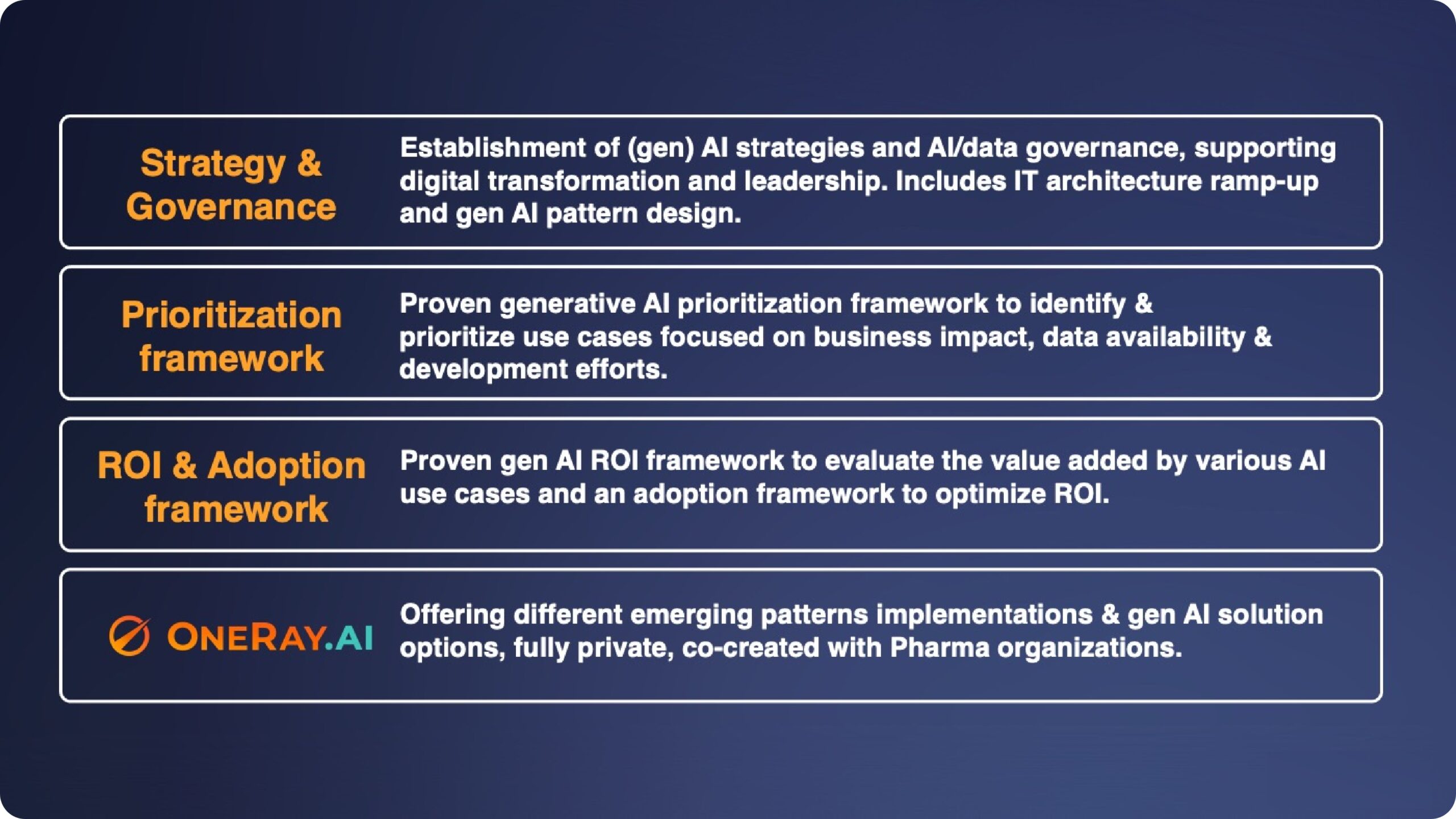
H2 2025 & 2026 AI Implementation Opportunities: Strategic Planning for Proven ROI in Pharma, Biotech & Medtech
As life sciences organizations allocate H2 2025 and 2026 budgets, the question isn't whether to invest in generative AI - it's how to implement it strategically for maximum impact.The...